How do chloroplasts maintain energy efficiency?
Although the chloroplast is the key energy harvester and producer in a plant cell, its demand for ATP is also extremely high. Illumination increases chloroplast ATP concentration instantly, but it drops to a basal level very quickly after illumination stops. Our results suggest that there was a need to restrict ATP consumption in mature chloroplasts in the dark.
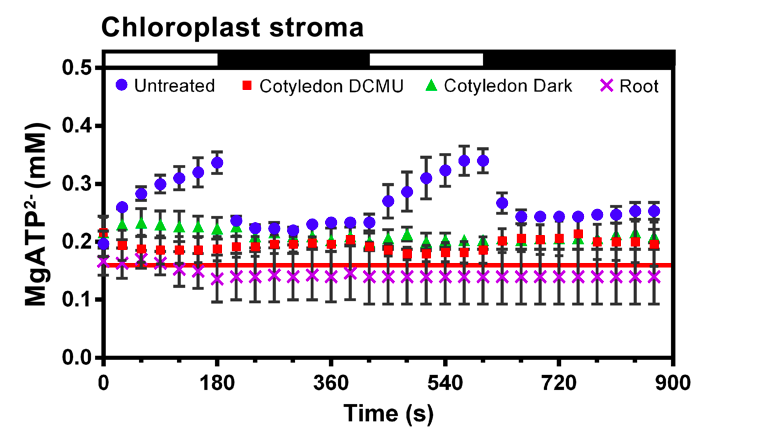
We expressed ATP transporters in the mature plant cells. We found that the cytosolic ATP was being exhausted by the chloroplasts. This finding explains why ATP transporters are only present in the young seedlings. During early developmental stage, young chloroplasts required exogenous ATP for biogenesis. When the chloroplasts were fully developed and became self-sustainable, the ATP transporters had to be downregulated so that the energy produced through photosynthesis during daytime would not be deliberately consumed by chloroplasts at night.
A paper published in Nature in 2015 (524:366–369) showed that chloroplasts in unicellular diatoms can import cytosolic ATP to support carbon fixation.We propose that during the evolution of unicellular to multicellular photosynthetic organisms, the flux of ATP between the cytosolic and stromal compartments was restricted by the downregulation of the nucleotide transporters (NTT) across the chloroplast inner membrane. Unlike diatoms, which are self-sufficient, source cells in higher plants have to restrict energy consumption at night; thus, higher plants can conserve more carbon for export to the sink tissues to support plant growth. Wasteful energy consumption must be avoided in the dark.
Click here to view the press release:
An HKU-led team of international plant scientistsreveal how chloroplasts maintain energy efficiency
Flow of reducing equivalents between chloroplasts and mitochondria
During photosynthesis, the photosystem generates both NADPH and ATP at a ratio of 0.77, of which 0.66 was consumed by CO2 fixation. Excess reducing equivalents carried by surplus NADPH can be exported to the cytosol through the malate-oxaloacetate shuttle in the form of malate and results in the build-up of malate in leaf cells during daytime.
In C3 plants, photorespiration concurrently happens with photosynthesis. For every 3 fixed CO2 molecules, one O2 molecule is mistakenly fixed by Rubisco in chloroplasts. The recycling of the photorespiratory product involves eight enzymatic steps in chloroplasts, mitochondria, and peroxisomes. Hence, photorespiration has been regarded as a wasteful process due to the additional consumption of ATP and liberation of CO2 in mitochondria in the cycle. During photorespiration, NADH is generated in the mitochondria through the action of glycine decarboxylase.
In mitochondria, both the malate-OAA shuttle and photorespiration have been proposed to provide reducing equivalents to the mitochondrial electron transport chain for ATP production. However, the direction of the malate-OAA shuttle across the mitochondrial membrane during photosynthesis has been a matter of debate.
By employing a NADPH and a NADH/NAD+sensor, we examined in planta dynamic changes in the NADPH pools and NADH/NAD+ratio upon illumination. We found that photorespiration supplies a large amount of reducing equivalents to mitochondria during photosynthesis, which exceeds the NADH-dissipating capacity of the mETC. Consequently, the surplus NADH must be exported from the mitochondria to the cytosol through the mitochondrial malate-OAA shuttle. The results were published in Nature Communications.
Click here to view the press release:
Revisiting energy flow in photosynthetic plant cellsHow do guard cell chloroplasts obtain energy?
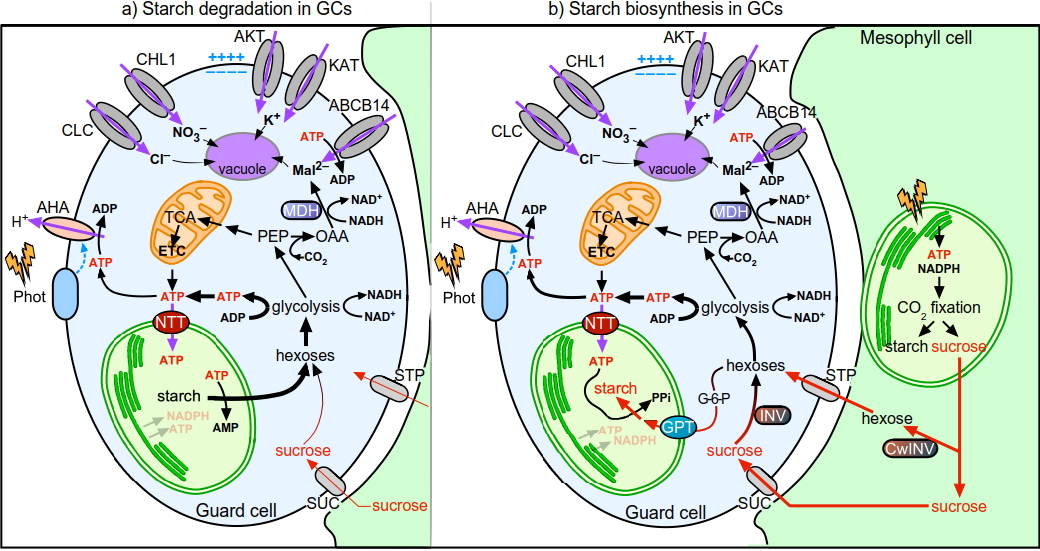
1. Sizes: The diameter of GCC is ~40% of MCC, and therefore the volume is around 1/16.
2. Photosynthesis: The major function of MCC is photosynthesis, whether the major function of GCC isto serve as a starch reservoir for stomata movement as GCC has low photosynthetic activity.
3. Source of stromal ATP: MCCs generate ATP from photosynthesis whereas GCCs mainly import cytosolic ATP provided by mitochondria through the plastidial ATP/ADP translocator NTT1. A fraction ofthe imported ATP is directly used to energise starch synthesis,which, in turn,is essential for light-regulated stomatal movements.
4. CO2 fixation: MCCsfix CO2 mainly by Rubisco, whereas in GCs, anaplerotic CO2 fixation in cytosol is the main pathway of carbon assimilation. Guard cells (GCs) and mesophyll cells (MCs) have comparable amount of cytosolic MDH and PEPc, but GCs only contain 1/16 Rubisco of MCs. CO2 was fixed into Mal2– at high rates in GCs, whereas in MCs, CO2 was enriched in 3-PGA and sucrose in the light and Mal2– only in the dark.
Our work demonstrates that GCCs and MCCs greatly differ in the way they obtain energy and carbon skeletons. GC photosynthesis is poorly active, and mitochondria are the major source of ATP for GCs. Unlike MCs, GC metabolism mainly favours sucrose and starch degradation upon transition from dark tolight. Based on our findings, we propose a model of the roles of GCCs in stomatal opening and their energy and carbon metabolism (Figure). The results were published in NatureCommunications.